Etern Therapeutics announced that the U.S. FDA has granted orphan drug designation to the YAP/TEAD inhibitor ETS-006 for treating patients with malignant mesothelioma. Etern has not yet submitted the IND application in either China or the U.S.
Hippo pathway dysregulation can lead to diseases like cancer. Drug development focuses on targeting Hippo kinases, YAP/TAZ levels, and YAP-TEAD interactions, aiming to disrupt YAP-TEAD activity and inhibit disease progression.
 |
| The core Hippo pathway |
ETS-006
ETS-006, an orally available YAP/TEADs PPI inhibitor, is discovered by Etern Therapeutics. YAP, with TEAD transcription factors, drives Hippo pathway signaling and regulates cancer progression. Its overactivation promotes metastasis, immune evasion, and drug resistance. Inhibiting YAP/TEAD interactions effectively suppresses YAP’s transcriptional activity.
In 2022, Etern filed a patent (WO2023155927) for the YAP/TEAD inhibitors. The structure of example 124 is shown below.
| Structures in the patent |
At the AACR 2024, Etern presented the preclinical results of ETS-006, the second candidate, which could completely disrupt the interaction of YAP/TEADs compared to the previously reported ETS-003. At that time, Etern also reported on ETS-005, which is a highly selective inhibitor of TEAD4 palmitoylation.
YAP/TEAD inhibitor
Results from two early clinical trials indicate potential preliminary efficacy and toxicity in specific cancers and mutations. Although some research on this target has been discontinued, other studies are still ongoing, and more competitors are entering this clinical field.
VT3989
VT3989, discovered by Vivace Therapeutics, is currently undergoing a phase 1/2 study (NCT04665206, 2021.03) in patients with solid tumors, including advanced mesothelioma (MM).
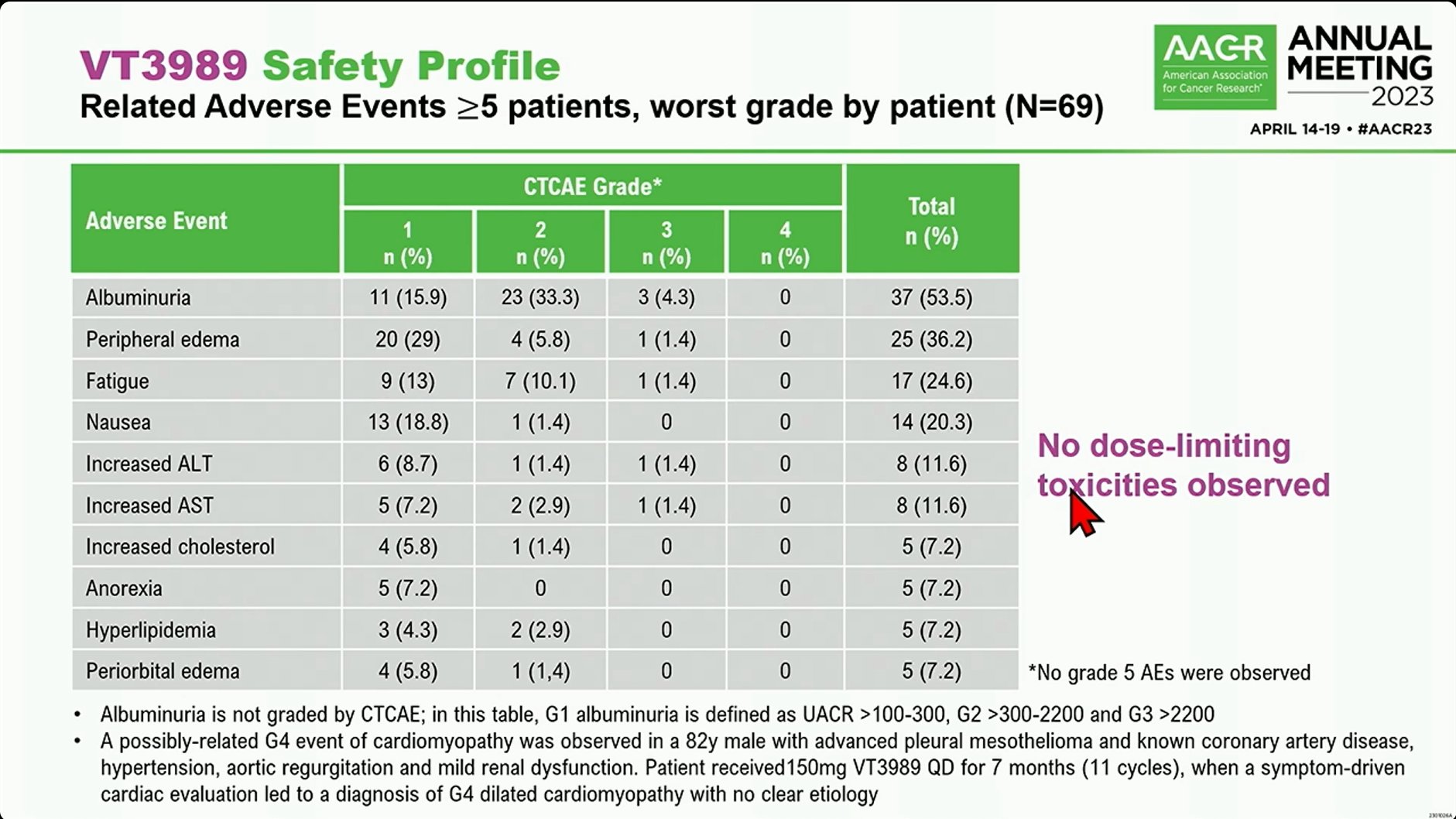
In 2023, Vivace reported the preliminary results of this trial. Seven pts (6 MM and 1 NF2m sarcoma) achieved PRs with 4 confirmed, and 3 unconfirmed. 3 PRs in MM pts are ongoing for up to 18+ months. The clinical benefit response rate (PR+SD>8 weeks, per protocol) in MM pts is 57%. And, there were 7 possibly related G3 AEs (fatigue, ALT, AST, dehydration, dyspnea, hypotension, and peripheral edema) and 1 G4 cardiomyopathy reported.
 |
| FIH phase I trial of VT3989 |
IAG933
IAG933, discovered by Novartis, is a YAP1/WWTR1(TAZ)-panTEAD protein-protein interaction inhibitor. It is currently under investigation in a phase 1 trial (NCT04857372, 2021.10) for patients with mesothelioma, NF2/LATS1/LATS2 mutated tumors, and tumors with functional YAP/TAZ fusions. The administration schedules were designed for continuous dosing, as well as for dose holiday periods as the exploration in Vivace's trial. No results from this trial have been reported yet.
BPI-460372
BPI-460372, discovered by Betta Pharma, is a novel small molecule that covalently and irreversibly binds to the cysteine residue in the TEAD palmitoylation pocket to block the TEAD auto-palmitoylation. Betta commenced a phase 1 trial (NCT05789602, 2023.03) in patients with mesothelioma, epithelioid hemangioendothelioma, or other solid tumors with NF2 defects, YAP/TAZ fusion, LATS1/2 mutations, and other Hippo signaling pathway abnormalities in March 2023.
BGC515
BGC515 is a BridGene-developed, orally-administered, covalent TEAD inhibitor, which is currently under investigation in the phase 1 trial (NCT06452160, 2024.06) in patients with mesothelioma (MM), epithelioid hemangioendothelioma (EHE), or other advanced solid tumors.
SW-682
SW-682, discovered by SpringWorks Therapeutics, is a pan-TEAD small molecule inhibitor that blocks TEAD-dependent transcription by binding to the palmitoylation pocket of all TEAD isoforms. SpringWorks initiated the phase 1 trial (NCT06251310, 2024.07) of SW-682 in patients with mesothelioma and other advanced solid tumors with NF2 mutations or other Hippo pathway mutations.
ISM6331
ISM6331, a novel and potent pan-TEAD inhibitor with excellent inhibition against TEAD1/2/3/4 was identified leveraging an AI generative mode and was developed by InSilico Medicine. The candidate is undergoing a phase 1 trial (NCT06566079, 2024.08) in patients with mesothelioma and other solid tumors.
TY-1054
TY-1054, an oral TEAD inhibitor targeting the Hippo pathway discovered by TYK Medicines, shows promising antitumor efficacy and safety in early studies. IND submissions to the FDA and NMPA were anticipated by Q4 2023, but they did not meet the deadline.
IK-930
Ikena discontinued the development of IK-930, a TEAD1 selective palmitoylation inhibitor, earlier this year due to limited activity observed as monotherapy in the phase 1 trial even after the formulation optimization.
Comments