Simcere/Zenshine/Xianxiang has submitted the IND application for the CDH6 antibody-drug conjugate (ADC) SIM0505 in China. According to the company statement, this ADC is being developed as a potential treatment for malignant tumors, such as ovarian and renal cancer.
CDH6 is a type II classical cadherin, also known as K-cadherin, consisting of 790 amino acids, located in the lateral basement membrane of epithelial cells, where it mediates calcium-dependent cell-cell adhesion. CDH6 is expressed in various cancer types, including kidney, ovarian, and thyroid cancers.
| CDH6‐activated αIIbβ3 crosstalks with α2β1 to trigger cellular adhesion |
SIM0505
SIM0505 is a CDH6-targeting antibody-drug conjugate (ADC) by linking a CDH6-specific monoclonal antibody, which binds selectively to tumor cells, with camptothecin toxoid molecules. The company described the product is designed by combining the tumor targeting of antibodies with the high-efficiency killing effect of toxin molecules, while avoiding the defects of low curative effect of the former and excessive toxic side effects and poor drug-making performance of the latter.
In 2023, Simcere filed a patent (CN202310420429.5) for antibody-drug conjugates targeting CDH6 and their use. The structural form is shown below, compared with Daiichi Sankyo's R-DXd. The examples (DAR: 7.5-8.2) in the patent demonstrated comparable tumor inhibition activities to R-DXd.
| Structures in patent |
CDH6 targeted candidates
CDH6 is an established target in the therapeutic landscape but has relatively few competitors developing targeted therapies. Daiichi Sankyo's R-DXd is currently the leading candidate in clinical development, with multiple clinical results presented at recent medical congresses.
Raludotatug Deruxtecan (R-DXd, DS-6000a)
Raludotatug Deruxtecan is an antibody-drug conjugate (ADC) that targets CDH6. It was discovered by Daiichi Sankyo using DXd ADC technology. This product consists of a humanized IgG1 monoclonal antibody against CDH6, linked to eight payloads of a topoisomerase I inhibitor, derived from exatecan (DXd), through cleavable tetrapeptide linkers. Currently, Raludotatug Deruxtecan is being developed under a collaborative agreement with Merck.
In 2023, Daiichi Sankyo presented the clinical results of the phase 1 trial in patients with heavily pretreated platinum-resistant advanced ovarian cancer. The phase 1 study assessed the tolerability of R-DXd at 1.6 to 9.6 mg/kg, and 8.0 mg/kg was determined to be the maximum tolerated dose.
In the Part B of the study, patients received R-DXd at 4.8 to 8.0 mg/kg. 91.7% of patients had platinum-resistant disease with four median prior lines of systemic therapy (range, 1-13) including bevacizumab (68.3%) and a PARP inhibitor (65%). A confirmed ORR of 46% was observed in the subgroup of 50 patients, which included 1 CR and 22 PRs. A DCR of 98% was observed. mDOR was 11.2 months and mPFS was 7.9 months. Treatment discontinuations due to TEAEs occurred in 15% of patients. Grade 3+ TEAEs occurred in 51.7% of patients. The most common grade > 3 or higher TEAEs occurring in patients were anemia (18.3%), decreased neutrophil count (11.7%), decreased platelet count (5.0%), fatigue (3.3%), nausea (1.7%), vomiting (1.7%), diarrhea (1.7%) and decreased appetite (1.7%). Two grade 2 ILD events were confirmed as treatment-related across the 4.8 to 6.4 mg/kg doses. Two grade 5 treatment-related ILD events were reported at the 8.0 mg/kg dose.
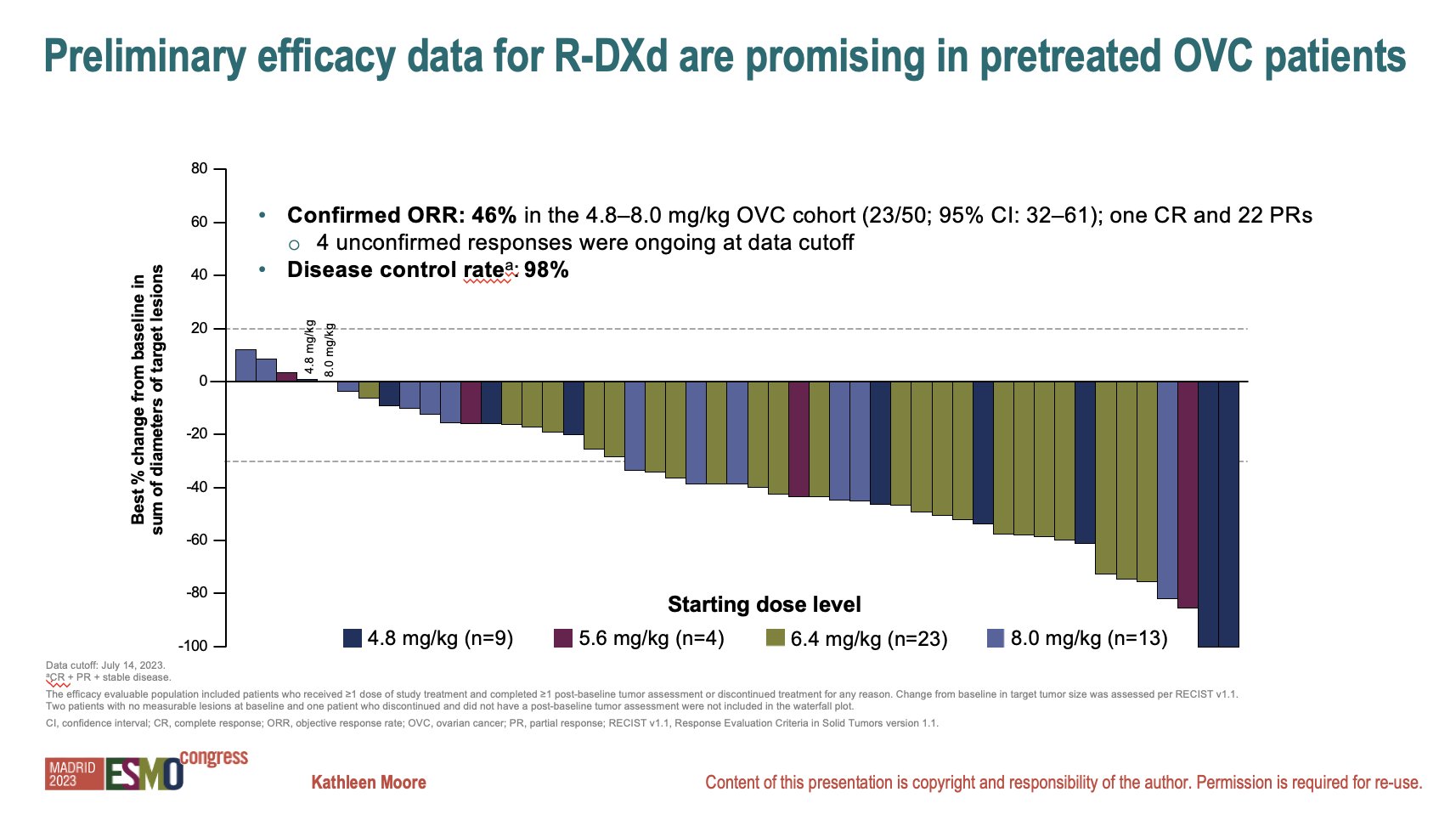 |
| Results of the phase 1 study |
CUSP06/AMT-707
CUSP06/AMT-707 is a CDH6-targeted ADC discovered by OnCusp Therapeutics and Multitude Therapeutics. It consists of a humanized anti-CDH6 IgG1 monoclonal antibody, an enzyme-cleavable linker, and exatecan with a drug-to-antibody ratio (DAR) of 8. The company is recruiting patients in the first-in-human phase 1 study in the United States.
| CUSP06 |
CUSP06 demonstrated comparable efficacy to R-DXd in the preclinical study. The company also emphasized the superior efficacy of the CDH6-low PDX model.
ATG-106
ATG-106 is an anti-CDH6×CD3 BsAb discovered by Antegene for ovarian cancer and kidney cancer. According to the company website, the product is under research in the in vivo efficacy stage.
HKT288
HKT288 is an optimized CDH6-targeting DM4-based antibody-drug conjugate (ADC) developed for the treatment of these diseases, which was discovered by ImmunoGen and developed by Novartis. The product was discontinued after the phase 1 trial. Novartis cited the termination of the phase 1 trial due to two suspected neurologic adverse events related to the drug. Due to the early termination, the MTD and efficacy data were not concluded and reported.
Comments