China's CDE has cleared the IND application for WGI-0301/HC0301, which is a lipid nanoparticle (LNP) formulation of an AKT-1 antisense oligonucleotide that binds to AKT-1 mRNA discovered by WhiteOak, a subsidiary of Haichang Biotech. This clearance is for use in patients with hepatocellular carcinoma. The product (RX-0301), along with WGI-0201/HC0201 (RX-0201) and WGI-0047 (RX-0047), was licensed from Rexahn Pharmaceuticals (now Ocuphire Pharma) in 2018.
WGI-0301
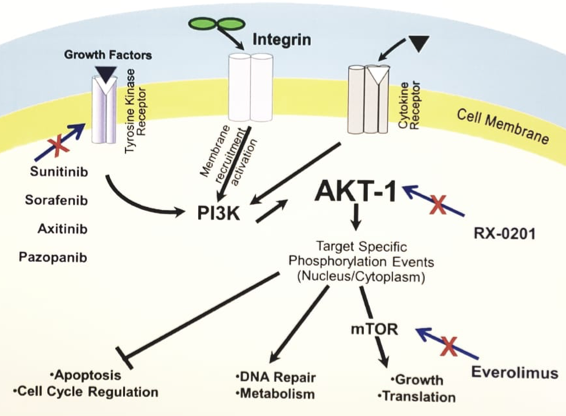
X-0301 is a nano-liposomal formulation of RX-0201, an antisense oligonucleotide compound designed to enhance delivery to target mRNA encoding Akt-1. RX-0201 works by binding to the mRNA, thereby inhibiting its transcription and the subsequent production of Akt-1 protein.
 |
| RX-0201 |
Rexahn explored the clinical use of RX-0201 in patients with pancreatic cancer and renal cell cancer before 2018. The median PFS in evaluable patients with mRCC treated with RX-0201 in combination with everolimus was 4.9 months, which is comparable to the FDA-approved use of everolimus alone (4.9 months). No further data has been reported regarding RX-0201 since then.
Haichang and WhiteOak presented preclinical data on WGI-0301, either alone or in combination with Lenvatinib, Sorafenib, or Cabozantinib, using a murine tumor model for hepatocellular carcinoma. The tumor growth inhibition percentage (TGI) for WGI-0301 alone was 46.16%. When combined, it also exhibited a significant inhibitory effect on angiogenesis, with rates of 66.05%, 86.81%, and 75.89%, respectively. These rates were higher compared to the single-drug groups of Lenvatinib, Sorafenib, or Cabozantinib, which had TGI rates of 50.84%, 55.68%, and 47.84%,
Haichang initiated a phase 1 trial of WGI-0301 in patients with solid tumors in August 2022 in the United States, following the approval of the IND application by the US FDA. Additionally, the orphan drug designation has already been granted for HCC in the US. No data on this trial has been reported thus far.
Phase 2 trial in HCC
After evaluating the results of the phase 1 trial of WGI-0301 alone in solid tumors, Haichang initiated a phase 2 trial specifically focusing on WGI-0301 in combination with sorafenib (not lenvatinib) for patients with hepatocellular carcinoma (HCC). In the press release in 2022, they announced their intention to commence the phase 2 trial in combination with PD-(L)1 inhibitors or sorafenib simultaneously in both China and the United States.
The administration schedule for WGI-0301 in combination with sorafenib differs from that of WGI-0201/RX-0201. Instead of a daily dose for 14 days followed by 7 days off, it involves a weekly dose. They estimate to enroll 60 patients in 3 groups: two groups receiving different doses of WGI-0301 combined with sorafenib, and one group receiving sorafenib alone.
----------------------------------
WGI-0301 is the first Class 1 drug candidate to validate Haichang's Quaternary-Tertiary Lipoamine Nanoparticle Nanoscale Gene Delivery Platform, as well as the validation of AKT-1 antisense in the treatment of HCC. We will continue to monitor its progression closely.
 |
| Quaternary-tertiary lipoamine nanoparticle |
Comments