Boehringer Ingelheim filed an IND application for BI 1819479, an ATX inhibitor that inhibits lysophospholipase. The US FDA granted an Orphan Drug Designation to BI 1819479 for the treatment of idiopathic pulmonary fibrosis in November 2023.
BI 1819479
Boehringer Ingelheim has completed the phase 1 trials of BI 1819479 in healthy participants. In 2024, Boehringer Ingelheim initiated the phase 2 trial in patients with Idiopathic Pulmonary Fibrosis (IPF). The study consists of 3 doses and 1 placebo group, aiming to enroll 300 patients. Annual rate of decline in Forced Vital Capacity (FVC) is the primary endpoint.
As stated by Boehringer Ingelheim, BI 1819479 is an investigational compound that may address pulmonary fibrosis—a scarring of the lung tissue that negatively impacts lung function—associated with IPF, a type of interstitial lung disease. And, the FDA granted Orphan Drug Designation to BI 1819479 based on the availability of preclinical data.
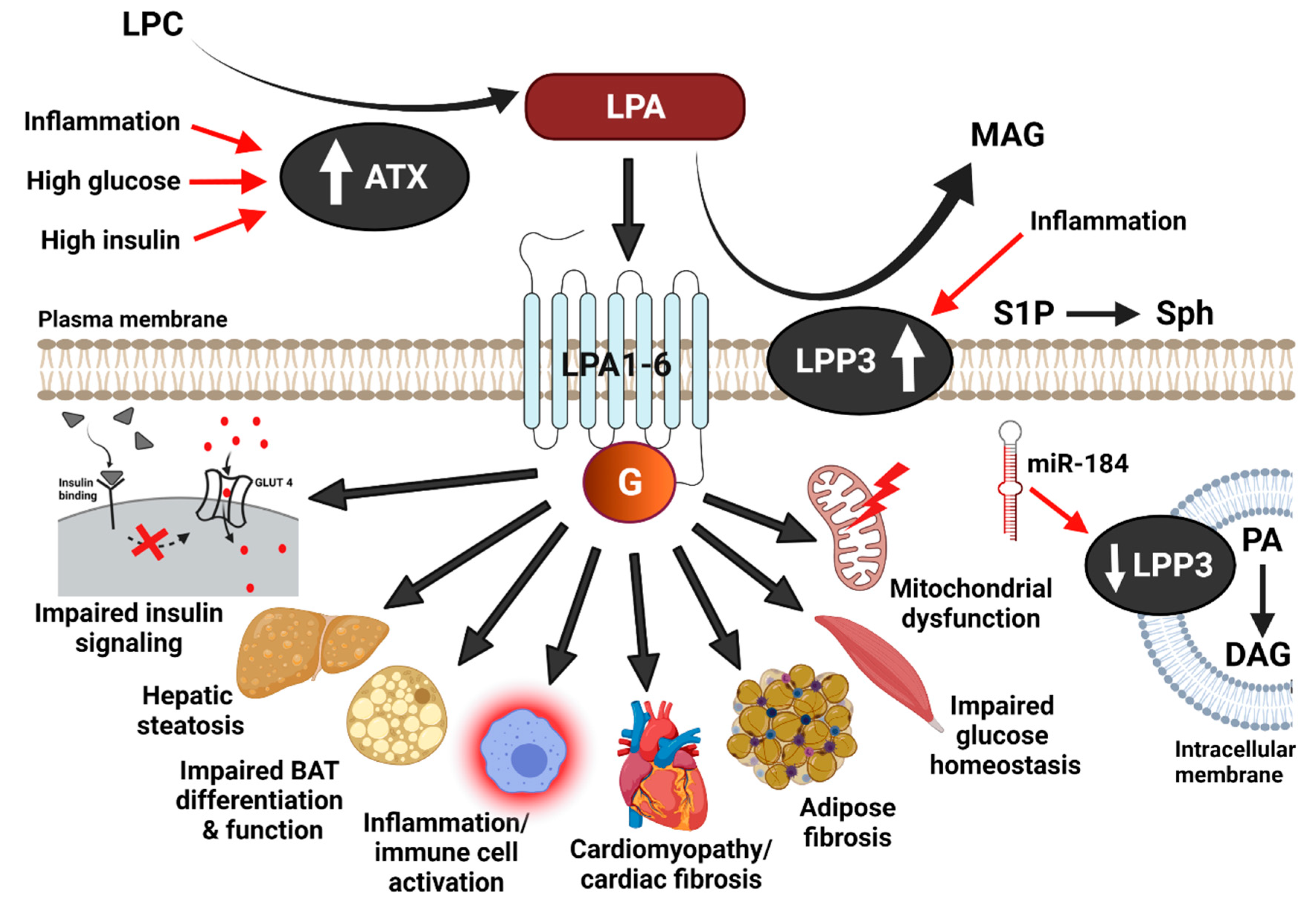 |
| Metabolic effects of the ATX-LPA-LPP3 axis |
In 2019, Boehringer Ingelheim filed a patent (WO2021013830) covering N-methyl, N-(6-(methoxy)-pyridazin-3- yl) amine derivatives as ATX inhibitors for the treatment of idiopathic lung disease (IFF) or systemic sclerosis (SSc). Examples and reference compounds are listed below. LPA reduction in vivo is determined by measuring the plasma concentration of LPA after oral dosage of the compounds. Boehringer Ingelheim did not report the result of Ziritaxestat but only gave the number of two compounds in the patent of Ziritaxestat. Boehringer Ingelheim's BI-2545, an ATX inhibitor, was also tested for its ability to reduce LPA levels.
| Examples in the patent |
ATX inhibitors
Ziritaxestat
Galapagos and Gilead discontinued the phase 3 trial of Ziritaxestat in IPF, based on the recommendations of the Independent Data Monitoring Committee (IDMC). Ziritaxestat gained significant attention as a potential first-in-class treatment.
 |
| Mean (±SEM) plasma exposure of Ziritaxestat and LPA 18:2 reduction |
In 2023, Galapagos reported the results of the 2 phase 3 trials. In the 2 clinical trials, ISABELA 1 and ISABELA 2, there was no reduction in the 52-week rate of decline for forced vital capacity (a measure of lung function) in the 2 ziritaxestat groups vs placebo, and combined data from both trials showed all-cause mortality rates were numerically higher for ziritaxestat than placebo. Therefore, the trials were stopped early. In ISABELA 1, all-cause mortality was 8.0% with 600 mg of ziritaxestat, 4.6% with 200 mg of ziritaxestat, and 6.3% with placebo; in ISABELA 2, it was 9.3% with 600 mg of ziritaxestat, 8.5% with 200 mg of ziritaxestat, and 4.7% with placebo.
Cudetaxestat (BLD-0409)
BBT-877
Bridge Biotherapeutics acquired the exclusive global license for BBT-877 from LegoChem Biosciences in May 2017. In 2019, Boehringer entered a deal with Bridge Biotherapeutics to develop the drug candidate, BBT-877. The deal was terminated in 2020 after the initiation of the phase 1 trial in healthy participants.
During the US-based Phase 1 clinical trial, the data on LPA inhibition in the multi-ascending dose study showed that 100mg and 200mg twice daily dosages induced LPA inhibition of up to 90%. In April 2023, Bridge dosed the first patients in the phase 2 clinical trial of BBT-877.
HW021199 (Discovered by a China-based company)
Likang Pharma has initiated the phase 2a study of HW021199, an ATX inhibitor, with the first patient-in occurring in January 2024.
Cambritaxestat (IOA-289)
iOnctura discovered Cambritaxestat, the only autotaxin inhibitor being developed in oncology. The phase 1/2 study is undergoing in patients with pancreatic cancer in 2022.
HNC664 (Discovered by a China-based company)
Henovcom initiated the phase 1b trial of HNC664, an ATX inhibitor, alone or in combination with paclitaxel and gemcitabine, for patients with pancreatic cancer in 2022.
Comments