China CDE has approved Glubio's GLB-001, a CK1α degrader, for the Investigational New Drug (IND) application in patients with myeloid neoplasm (AML/MDS). Glubio Therapeutics submitted the IND application on December 9th, 2023.
The FDA cleared the IND application for GLB-001 in April 2023, and the first-in-human trial (NCT06146257) was initiated in January 2024, focusing on patients with relapsed or refractory acute myeloid leukemia (R/R AML) or higher-risk myelodysplastic syndromes (R/R HR-MDS).
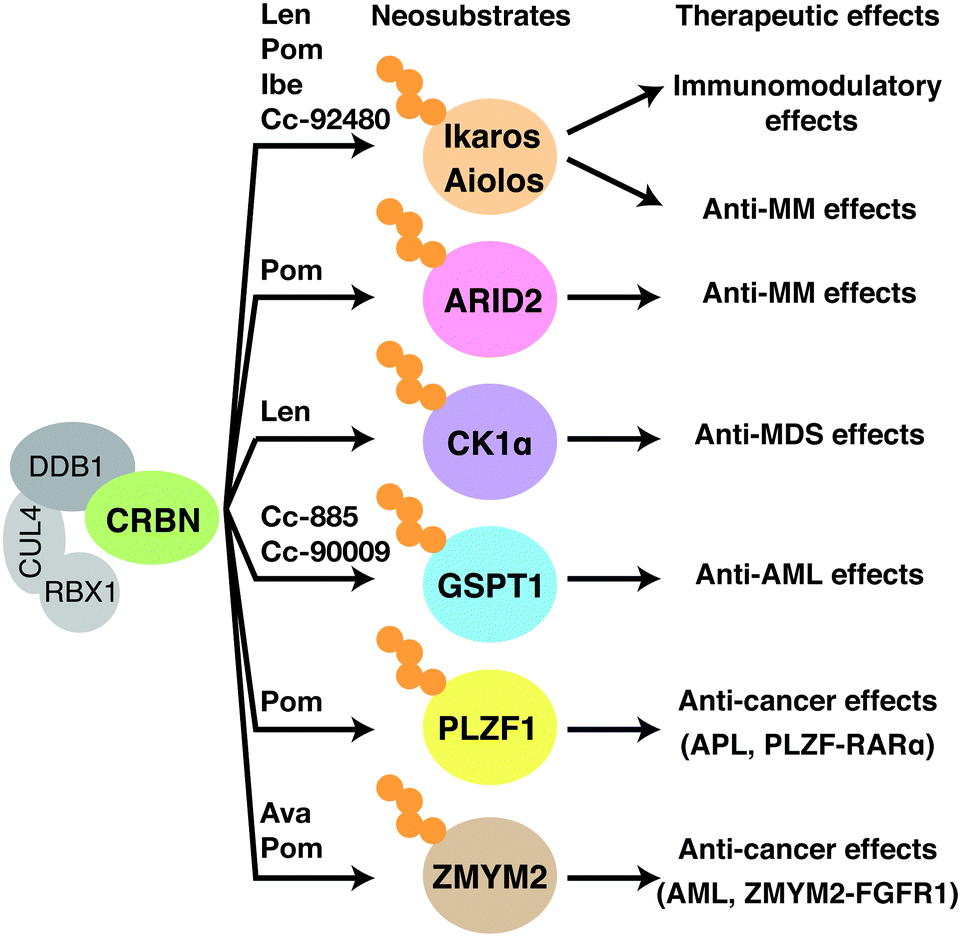
GLB-001 is a molecular glue targeting CK1α, different from their second degrader GLB-002, which is targeting Aiolos and Ikaros. Casein kinase 1 alpha (CK1α), encoded by gene CSNK1A1, is a ubiquitously expressed serine/threonine protein kinase in the CK1 kinase family. CK1α, as a key regulator of the Wnt/β-catenin pathway, directly phosphorylates β-catenin at Ser45 and thereby targets it for proteasomal degradation. CK1α was also known to regulate the protein stability of tumor suppressor p53 via modulating the activity of the MDM2/MDMX E3 ligase complex. Overexpression of CK1α in many types of human cancers was reported, however, the precise role of CK1α in the development of each respective tumor type has not been clearly elucidated. CK1α is a well-known neosubstrate of lenalidomide, an FDA-approved drug for the treatment of human multiple myeloma and lower-risk myelodysplastic syndromes with 5q deletion (del (5q) MDS). GSPT1, also known as eRF3a (eukaryotic release factor 3a), is a key translation termination factor that binds and activates eRF1 to mediate stop codon recognition and nascent protein release from translating ribosomes.
 |
| DOI: 10.1039/D2CS00116K |
In 2021, Glubio filed a patent (WO2022257897) covering the CK1α degraders with mild activity towards GSPT1 (Dmax 15%~30%) to avoid toxicity. The compounds were also tested using an AML cell line harboring p53 mutation such as KG-1a, to determine the GSPT1-mediated cytotoxicity in vitro.
Comments