On December 26, 2023, Leado Pharmatech filed an IND application for LD09163 a TRPA1 inhibitor, in China. According to their pipeline information on the website, LD09163 is specifically designed for patients with ulcerative colitis and Crohn's disease.
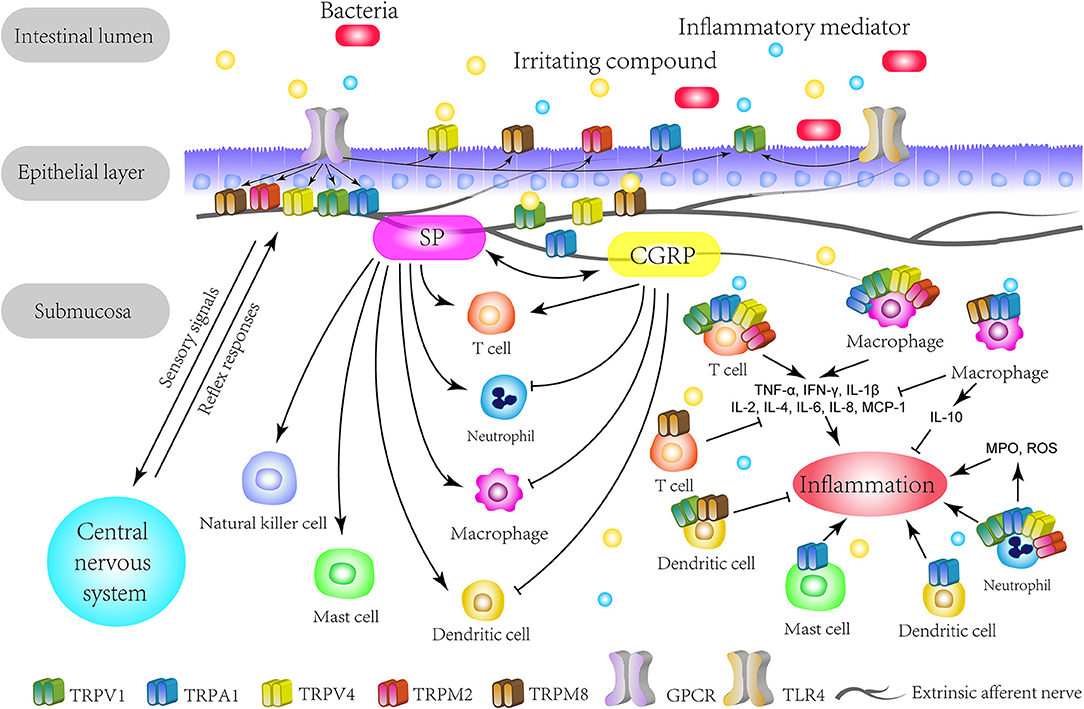
Companies such as Lilly have explored the use of their TRPA1 inhibitor for pain relief before. As reported by Yiding Chen, "In animal studies, mice with experimental colitis exhibited an increased TRPA1-mediated colonic neuropeptide release, while the experimental colitis appeared to be less severe after the inhibition of TRPA1 by the antagonist or genetic depletion."
 |
| https://doi.org/10.3389/fimmu.2020.00180 |
LD09163 is a novel ion channel drug developed by Leado Pharmatech for the treatment of Inflammatory Bowel Disease (IBD). After oral administration, it can selectively accumulate in the gastrointestinal tract, increasing drug concentration in target tissues and reducing systemic toxicity. Its performance in various efficacy models surpasses that of current clinical treatment drugs.
In 2018 and 2021, Leado filed patents (WO2020035040 and WO2022262657) to protect amine compounds acting as TRPA1 inhibitors. The compounds described in WO2020035040 were evaluated in animal models for pain relief, whereas those in WO2022262657 underwent testing in animal models for DNBS-induced and DSS-induced colitis.
Comments